Tag Archives: Krystexxa
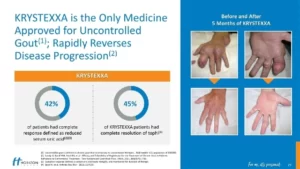
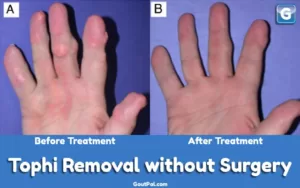
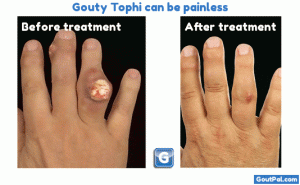
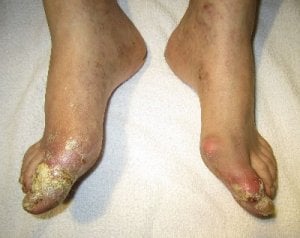
The Krystexxa blog brings you news and updates about pegloticase. Krystexxa is a brand of pegloticase, and this blog covers it’s development and availability. These articles support the Krystexxa (pegloticase) guidelines, where you can also find links to other forms of uricase.